
Publications
Increasing Global Research
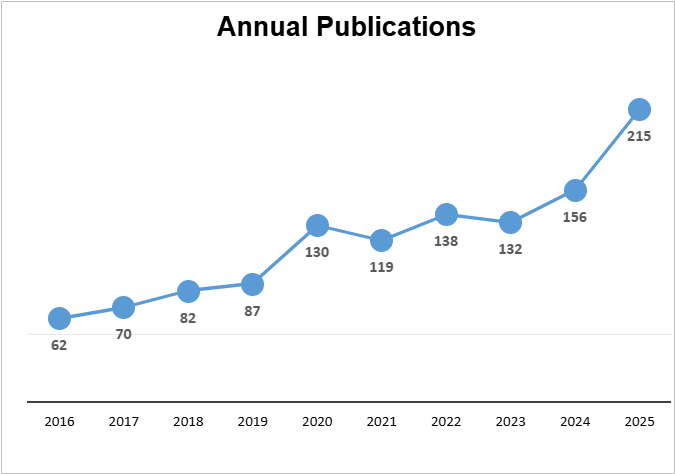
NACC has seen a significant increase in impactful research and publications. In 2025, 215 journal articles were published using NACC data (a 38% increase over the prior year).
The UDS continues to be utilized for clinical phenotypic data collection globally. Since the start of the U24 (June 2021) through March 2025, NACC has granted 100+ requests for the use of copyrighted NACC UDS forms to non-ADRC institutions worldwide, enabling them to conduct research at their own institutions. This highlights the value of the UDS for standardized data collection, including outside of the ADRC Program.
With each data file request, NACC records a "proposal", including a brief description of the project being undertaken. These proposals can lead to one or more publications. Click the button below to see all publications.
Keystone Papers
The National Alzheimer’s Coordinating Center (NACC) provides one of the most extensive resources for Alzheimer’s and dementia research. The Uniform Data Set (UDS) Version 3, developed with Alzheimer’s Disease Centers, offers standardized longitudinal clinical and neuropsychological data from thousands of participants. In addition, NACC’s Neuropathology dataset is one of the largest multi-center repositories of detailed postmortem findings, supporting both hypothesis-driven studies and new discoveries. Together, these resources give researchers a powerful foundation for advancing understanding of dementia and cognitive impairment.
- Chan KCG, Xia F, Kukull WA. NACC data: Who is represented over time and across centers, and implications for generalizability. Alzheimers Dement. 2025 Sep;21(9):e70657. doi: 10.1002/alz.70657. PMID: 40968249; PMCID: PMC12445991.
- Chan KCG, Dodge HH, Sano M, Au R, Craft S, Levey AI, Weintraub S, Kukull WA, Saykin AJ, Barnes LL. Comparable performance of the NACC Uniform Data Set version 3 neuropsychological test battery in assessing longitudinal cognitive change for African American and White participants. Alzheimers Dement. 2025 Nov;21(11):e70889. doi: 10.1002/alz.70889. PMID: 41206462; PMCID: PMC12596166.
- Zuelsdorff M, Abner EL, Balls-Berry JE, et al. Introducing socialdeterminants of health to the Alzheimer’s Disease Research Center network: development and implementation in the Uniform Data Set. Alzheimers Dement. 2025;21(5):e70279. doi:10.1002/alz.70279
- Weintraub, S., Besser, L., Dodge, H. H., Teylan, M., Ferris, S., Goldstein, F. C., Giordani, B., Kramer, J., Loewenstein, D., Marson, D., Mungas, D., Salmon, D., Welsh-Bohmer, K., Zhou, X.-H., Shirk, S. D., Atri, A., Kukull, W. A., Phelps, C., & Morris, J. C. (2018). Version 3 of the Alzheimer Disease Centers’ Neuropsychological Test Battery in the Uniform Data Set (UDS). Alzheimer Disease and Associated Disorders, 32(1), 10–17. 10.1097/WAD.0000000000000223
NACC’s Neuropathology dataset is one of the largest multi-center repositories of detailed postmortem findings, supporting both hypothesis-driven studies and new discoveries. NACC data show that Alzheimer’s disease often co-occurs with other pathologies such as cerebral amyloid angiopathy, TDP-43 (LATE), Lewy bodies, and vascular injury. These mixed pathologies are strongly linked to dementia and cognitive decline. Findings also highlight how participant recruitment shapes observed pathology profiles, underscoring the importance of NACC’s large, standardized autopsy dataset for advancing research.
- Woodworth DC, Nguyen KM, et al. Evaluating the updated LATE-NC staging criteria using data from NACC. Alzheimers Dement. 2024 Dec;20(12):8359-8373. doi: 10.1002/alz.14262
- Gauthreaux K, Kukull WA, Nelson KB, et al. Different cohort, disparate results: Selection bias is a key factor in autopsy cohorts. Alzheimers Dement. 2024;20(1):266-277. doi:10.1002/alz.13422
- Center's Database for the Rapid Assessment of Evolving Neuropathologic Conditions. Alzheimer Dis Assoc Disord. 2020 Apr-Jun;34(2):105-111. 10.1097/WAD.0000000000000380
- Teylan M, Mock C, Gauthreaux K, Chen YC, Chan KCG, Hassenstab J, Besser LM, Kukull WA, Crary JF. Cognitive trajectory in mild cognitive impairment due to primary age-related tauopathy. Brain. 2020 Feb 1;143(2):611-621. doi: 10.1093/brain/awz403. PMID: 31942622; PMCID: PMC7009602.
- Besser, L. M., Kukull, W. A., Teylan, M. A., Bigio, E. H., Cairns, N. J., Kofler, J. K., Montine, T. J., Schneider, J. A., & Nelson, P. T. (2018). The Revised National Alzheimer’s Coordinating Center’s Neuropathology Form-Available Data and New Analyses. Journal of Neuropathology and Experimental Neurology, 77(8), 717–726. 10.1093/jnen/nly049
NACC data are advancing AI models that improve dementia diagnosis. A 2024 Nature Medicine study showed that combining clinical and imaging data with AI support significantly improved neurologists’ diagnostic accuracy across multiple dementia types. A 2022 Nature Communications study demonstrated that deep learning models trained on clinical and imaging data could match or surpass expert assessments while providing interpretable results linked to neuropathology.
- Ramanan S, Akarca D, et al. The graded multidimensional geometry of phenotypic variation and progression in neurodegenerative syndromes. Brain. 2025 Feb 3;148(2):448-466. doi: 10.1093/brain/awae233
- Zhou X, Balachandra AR, et al. Adversarial Learning for MRI Reconstruction and Classification of Cognitively Impaired Individuals. IEEE Access. 2024;12:83169-83182. doi: 10.1109/access.2024.3408840
- Yew PY, Devera R, Liang Y, et al. Unraveling the multiple chronic conditions patterns among people with Alzheimer's disease and related dementia: A machine learning approach to incorporate synergistic interactions. Alzheimers Dement. 2024 Jul;20(7):4818-4827. doi: 10.1002/alz.13923
- Xue C, Kowshik SS, Lteif D, et al. AI-based differential diagnosis of dementia etiologies on multimodal data. Nat Med. 2024;30(10):2977-2989. doi:10.1038/s41591-024-03118-z
- Qiu S, Miller MI, Joshi PS, et al. Multimodal deep learning for Alzheimer's disease dementia assessment. Nat Commun. 2022;13(1):3404. Published 2022 Jun 20. doi:10.1038/s41467-022-31037-5
Recent studies using NACC and global cohorts have identified 75 genetic risk loci for Alzheimer’s, highlighting pathways involving amyloid, tau, and microglia. Research also shows that people with two copies of APOE4 almost universally develop Alzheimer’s pathology by age 65, with predictable biomarker changes and onset patterns.
- Andrews SJ, Boeriu AI, et al. Dementia risk scores, apolipoprotein E, and risk of Alzheimer's disease: One size does not fit all. Alzheimers Dement. 2024 Dec;20(12):8595-8604. doi: 10.1002/alz.14300
- Wang P, Lynn A, et al. Genome-wide association studies identify novel loci in rapidly progressive Alzheimer's disease. Alzheimers Dement. 2024 Mar;20(3):2034-2046. doi: 10.1002/alz.13655
- Fortea J, Pegueroles J, Alcolea D, et al. APOE4 homozygozity represents a distinct genetic form of Alzheimer's disease. Nat Med. 2024;30(5):1284-1291. doi:10.1038/s41591-024-02931-w
- Bellenguez C, Küçükali F, Jansen IE, et al. New insights into the genetic etiology of Alzheimer's disease and related dementias. Nat Genet. 2022;54(4):412-436. doi:10.1038/s41588-022-01024-z
In Lewy body dementia, higher baseline plasma levels of phosphorylated tau isoforms, specifically p-tau181 and p-tau231, are associated with worse cognitive performance and a faster decline on the MMSE, supporting their use as accessible biomarkers of clinical severity. In presymptomatic familial frontotemporal dementia (f-FTD), multimodal models integrating clinical assessments, brain imaging, and plasma neurofilament light (NfL) reveal genotype-specific sequences of biomarker and clinical progression, with atrophy and NfL emerging as optimal endpoints for prevention trials and clinical measures serving well in early symptomatic stages.
- Hammers DB, Eloyan A, et al. Longitudinal cognitive performance of participants with sporadic early onset Alzheimer's disease from LEADS. Alzheimers Dement. 2024 Dec 23. doi: 10.1002/alz.14439
- Polsinelli AJ, Johnson S, et al. Neuropsychiatric symptom burden in early-onset and late-onset Alzheimer's disease as a function of age. Alzheimers Dement. 2024 Aug;20(8):5481-5491. doi: 10.1002/alz.14042
- Staffaroni AM, Quintana M, Wendelberger B, et al. Temporal order of clinical and biomarker changes in familial frontotemporal dementia. Nat Med. 2022;28(10):2194-2206. doi:10.1038/s41591-022-01942-9
- Gonzalez MC, Ashton NJ, Gomes BF, et al. Association of Plasma p-tau181 and p-tau231 Concentrations With Cognitive Decline in Patients With Probable Dementia With Lewy Bodies. JAMA Neurol. 2022;79(1):32-37. doi:10.1001/jamaneurol.2021.4222
